Editor’s Note: This text course is an edited transcript of an Oticon Medical live webinar. Download supplemental course materials.
Dr. Ravi Sockalingam: Today I will be talking about implantable auditory technologies. My agenda includes middle ear implants, implantable bone conduction technologies, cochlear implants, and auditory brainstem implants. Although I work for Oticon Medical, we do not manufacture middle ear implants. However, I did include middle ear implants in this talk today because I think it is important to understand what these implants are, what they are used for, and the indications and contraindications of middle ear implants, especially those that are used in the United States.
Implantable bone conduction technologies, particularly percutaneous bone anchored hearing devices or osseointegrated hearing devices, are the mainstay of Oticon Medical’s business. I will spend a considerable amount of time today talking about percutaneous bone anchored hearing devices. There are some current developments in these bone conduction technologies that will shape the future of these technologies. I will be speaking about the different kinds of transcutaneous solutions as well. I will also touch briefly on cochlear implants to address some of the advances and what we can see in the near future as far as technology goes. I will also touch on auditory brainstem implants.
Middle Ear Implants
Med-EL Vibrant Soundbridge: Semi-Implantable
The MED-EL Vibrant SOUNDBRIDGE was originally developed by Symphonix, based out of San Jose, California. It was cleared by the Food and Drug Administration (FDA) about 14 years ago and is the most widely used middle ear implant. Over 1,000 implantations have been performed in the U.S. and Europe. It is based on electromagnetic stimulation, like most other middle ear implants.
It consists of an audio processor with an implanted coil on the inside. The audio processor (Figure 1) transmits the information transcutaneously to the implanted coil on the other side. That sound is then transformed into an electromagnetic signal by the coil that wraps around the implant. The implant itself is called a floating mass transducer (Figure 1), and it is attached to the junction between the incus and the stapes. The audio processor functions like a hearing aid by picking up the acoustic sounds and the vibrating ossicle or prosthesis and then transforms the acoustic energy into electrical energy, which drives the transducer.

Figure 1. Med-EL Vibrant Soundbridge audio processor and floating mass transducer. From www.medel.com
Studies out of the Netherlands in the late 1990’s show that patients are quite satisfied with the quality of the sound. They experience less feedback and less occlusion. The surgery for this is called incus vibroplasty because the floating mass transducer is crimped around the long process of the incus. This is an example of an implant on the ossicular chain that physically vibrates the ossicles, which then sends a vibration to the cochlear fluids.
Maxum: Semi-Implantable
Another popular implant is the Maxum. Like the MED-EL Vibrant SOUNDBRIDGE, the Maxum is also semi-implantable. In semi-implantable middle ear implants, you have the audio processor resting on the outside of the body, typically on the skin, and the transducer has to be implanted inside. This was first introduced by Ototronix in 2009. The indications are for adults over 18 years of age with bilateral moderate to severe sensorineural hearing loss. It is also based on electromechanical stimulation or electromagnetic stimulation.
The surgical procedure for the Maxum is minimally invasive. You can use a transcanal approach under local anesthesia. Surgeons will perform this surgery in their office or hospital. It takes about 30 to 45 minutes. The implant consists of a magnet attached to the incudostapedial joint. The device is then activated four weeks after surgery.
It has an MRI compatibility up to 0.3 Tesla, which is a very small number. For the most part, these patients cannot undergo MRI scanning. The surgery is reversible, which means you can remove the magnet. It is the least expensive middle ear implant.
Figure 2 shows the audio processor integrated with the transceiver coil, or magnetic coil. The acoustic energy is picked up from the outside. The pinna helps to direct the acoustic energy into the audio processor, which is then processed like a hearing aid according to the hearing loss and the requirements of the user. That acoustic energy is then transformed into electromagnetic energy by the transceiver coil, and the electromagnetic waves would then be generated into the middle ear. The middle ear implant picks up the electromagnetic waves and moves according to the strength of the electromagnetic wave.
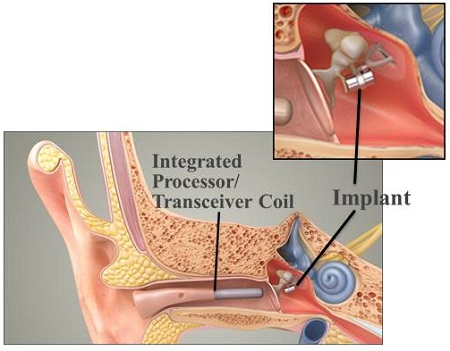
Figure 2. Maxum middle-ear implant. From ototronix.com
The distance between the transceiver coil and the implant is critical. The transceiver coil has to be close to the tympanic membrane for the implant to function adequately. The placement of the transceiver coil is important. At the top of Figure 2, you can see how the implant is imbedded in the incudostapedial joint.
Carina: Fully-Implantable
Carina was developed by Otologics, based in Boulder, Colorado. It is in its fourth generation, but I believe that Cochlear Corporation owns this company now. This middle-ear implant is fully implantable, which means that the processor is also inside; there is nothing visible outside the ear. This middle ear implant is indicated for mixed or conductive hearing loss, but the criteria for that indication is not very clear. For moderate to severe sensorineural hearing loss, there are very clearly defined criteria. It can be placed in people as young as 14 years old. It is based on electromagnetic stimulation.
Figure 3 shows the electromagnetic transducer attached to a laser-drilled hole in the bottom of the incus. This shows you the internal parts of the sound capsule, the microphone, and the transducer. The sensitive microphone picks up the sound and sits directly under the skin. We expect some transmission loss across the skin, so it has to be very sensitive to pick up the very soft sounds.
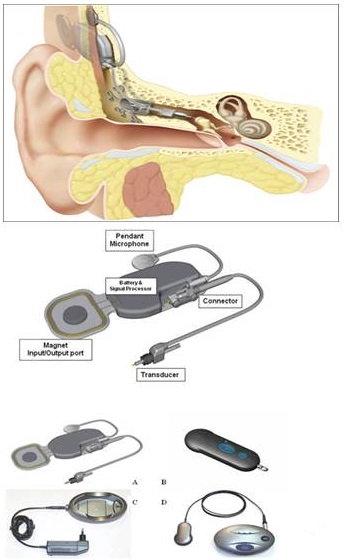
Figure 3. Carina middle-ear implant.
The electronic capsule encapsulates the speech processor, where all the processing is done. The magnetic port piece is interesting. You see, in Figure 3, the battery and the charger. Once the battery has been charged, you place it against the skull, and the internal capsule will pick up the signals. This charges the internal battery sitting inside the internal capsule. There is a remote control that can be used to change the volume or implant settings, and that would be placed on the skin over where the port is placed under the skin. This magnetic port is there to communicate with the remote control and the battery.
This implant requires a two-hour surgery under general anesthesia. The internal battery can last for 10 years, after which the electronic capsule must be replaced; not the transducer. There is no MRI compatibility, and it is not FDA approved. It does not exist in the U.S., but has been used overseas. Activation time is 8 weeks post implantation, and it costs about $15,000.
Envoy Esteem: Fully Implantable
The other fully implantable middle-ear implant is the Envoy Esteem implant, which is FDA approved. It has been available in the U.S. for about 10 years now. It was developed by Envoy Medical Corporation based in Minnesota. The indications are bilateral sensorineural hearing loss and speech discrimination greater than 50%. Unlike other middle-ear implants, which are based on electromagnetic stimulation, this is based on piezoelectric stimulation.
There are no microphones in this device. It consists of three parts: the sensor, speech processor, and driver (Figure 4). It uses the natural dynamics/vibrations of the ossicular chain. A sensor picks up the vibrations from the incus, and those vibrations are fed into the processor, like an internal hearing aid, which are then transformed into electrical signals, processed and amplified by the processor, which then turns the signal back into vibrational energy to the stapes. The vibrator is attached to the head of the stapes bone. The entire device is imbedded inside.
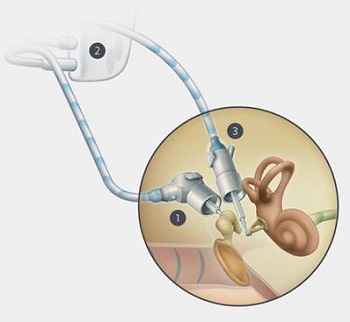
Figure 4. Envoy Esteem middle-ear implant. Source: envoymedical.com
The patient has to have a fairly large mastoid bone in order to accommodate this device. Additionally, the incus and stapes are disarticulated, and the long process of the incus is removed to eliminate any feedback vibrations. The activation time is about 45 days post implantation, and the battery is between four-and-a-half to nine years. If you are using it 16 hours a day, it will last about four-and-a-half years. If you are using it about eight hours a day, it will last nine years. If you are using it at 90 dB for 24 hours a day, you may still get two years and eight months out of the battery. When the battery runs out, you have to remove the whole processing unit, which contains the battery.
There is no MRI compatibility with this device. The disarticulation of the middle ear bones (incus and stapes) means there is no contact between the two bones. In some cases, if the device fails, there is a chance of restoring through the use of prosthesis, but in some cases, this may not be possible at all. There is also a possibility that the whole surgery may not be reversible. This is something you have to keep in mind.
This device costs around $30,000. This device is also surgeon-centric, because surgeons have to be certified to do this procedure, and they deal directly with the patient. The company then deals with the surgeon and the patient.
Implantable Bone Conduction Technologies
Let’s move into the bone conduction technologies. These are technologies that are used for mixed conductive hearing loss, as opposed to middle ear implants which are used for moderate to severe sensorineural hearing loss. There are two types of implantable bone conduction technologies: percutaneous bone anchored hearing devices and transcutaneous bone anchored solutions. The transcutaneous solutions can be further broken down into active, where the vibrator or the transducer is implanted inside, or passive, where the magnet is implanted but the vibrator is still outside.
Direct Drive Versus Skin Drive
Two kinds of bone conduction devices are direct drive and skin drive. Direct drive means the transducer that drives the cochlea is on the inside, but the source of vibration is still located outside. Skin drive has an implanted magnet, but the processor still sits outside. The down side of skin drive would be the attenuation of sounds across the skin.
Skin drive examples include the Sophono and the BAHA Attract. They are called passive transcutaneous bone conduction devices. It is similar to wearing a vibrator on a headband or a bone anchored sound processor on a Softband. The processors press against the temporal bone. The outcomes from these devices are very similar.
One the other hand, the direct drive is a percutaneous solution that drives vibrations straight through to the skull. These transcutaneous active solutions have vibrators that are imbedded inside, very close to the cochlea. There is little transmission loss and better high-frequency gain compared to skin-drive devices.
Percutaneous bone anchored hearing devices are what people commonly call Baha. Baha is a proprietary term owned by Cochlear Corporation, so we use bone anchored hearing systems (BAHS) or bone anchored hearing devices. The Cochlear Baha and the Oticon Medical Ponto are percutaneous bone anchored solutions. These devices are meant for unilateral, profound, sensorineural hearing loss, conductive and mixed hearing loss, or if patients have recurring otorrhea which prevents them from wearing traditional air conduction hearing aids.
Contraindications are that bone conduction thresholds cannot exceed 55 dB hearing level. Speech discrimination should be greater than 60%. If it is less, there will be a problem. In the United States, we do not implant children under the age of five years. In places where there is poor hygiene, this technology is not ideal.
Bone Anchored Hearing
Bone anchored hearing systems provide the most efficient transfer of vibration to the skull and into the cochlea. They deliver the best audiological outcomes when used following the correct indications. The surgery is straight forward, quick, and is typically performed under local anesthesia. It is also reimbursable.
In this system, the sound processor in on the outside of the skull, and the implant is imbedded into the temporal bone. The vibrations are sent to the skull, and those vibrations will then stimulate both cochleae through direct bone conduction.
What happens if one side has profound sensorineural hearing loss while the other has a functioning cochlea? This device can then act like a CROS hearing aid, except that you do not have a hearing aid sitting in one or both ears. The vibrations are sent to both cochleae, but the non-functioning cochlea will not receive those signals. The device is attached to an abutment, which is internally attached to an implant that is imbedded in the skull.
For many years, the Baha was the only device available. In 2009, Oticon Medical introduced the Ponto system (Figure 5). This coupling system, including the abutment and implant, functions like a bridge between the sound processor and the abutment. The sound processor attaches to the abutment, which is kept in place with a screw, and the implant is imbedded in the skull. The sound vibrations are picked up from the microphone outside and are transformed into mechanical vibrations that are transmitted along the abutment and implant into the temporal bone of the skull.

Figure 5. Ponto percutaneous bone anchored hearing system.
Figure 6 is a close up on the abutment and implant. This system is supported by the skin into the bone.
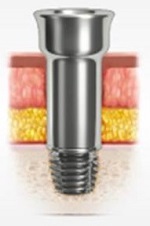
Figure 6. The Ponto abutment attached to implant.
Different abutment lengths are used for different thicknesses of the skin or connective tissue (Figure 7). The longer abutments enable surgeons to use minimally invasive surgical techniques. Back when surgical procedures were emerging, the skin was cut and tissue under the skin was removed. Currently, many surgeons use a linear incision or biopsy punch technique where they remove very little tissue, making it less invasive. Surgery time has been shortened considerably. The outcome is very cosmetically appealing, from post-op to processor loading. Figure 8 shows examples of what the system looks like with both the processor on and off the abutment.
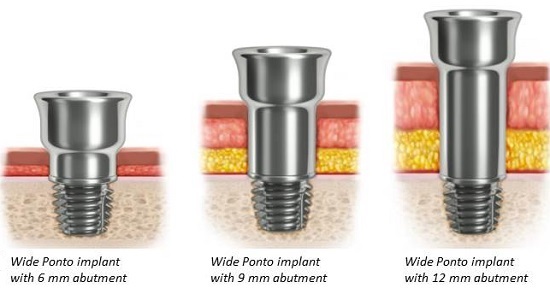
Figure 7. Various abutment choices for different skin/tissue compositions.
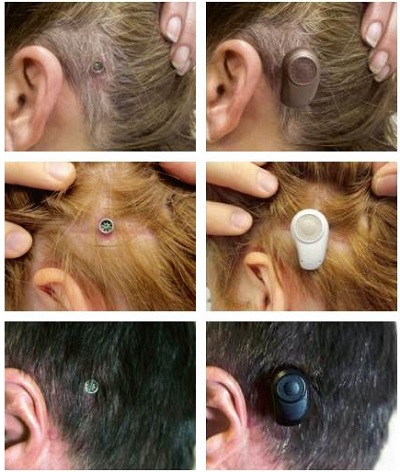
Figure 8. Outcomes of tissue preservation surgery. Abutment alone is shown on the left, with processor attached on the right.
With improved surgical techniques, we are seeing better cosmetics, less numbness, quicker healing, minimal scar tissue, and a reversible procedure; you can take the abutment out and leave the implant in. The implant is completed osseointegrated (fused) with the bone and will cause no problems for the patient. You can take the abutment off and the skin will grow over it. In that sense, it is completely reversible.
Ponto Pro Sound Processor
Figure 9 is a close-up of the Ponto Pro sound processor. It has all the features of an advanced hearing aid, including program selection, tamper-proof battery door, volume control and an inlet for programming. There is a large push button for the patient to change the programs. We have water and dirt resistance through nano coating with an IP57 classification. There is a safety line, which is important for children. The metal spring coupling system makes it durable and easy to snap on and off, as well as the tamper-resistant battery door and low-battery warning system. These digital devices are programmed using a similar interface and process as hearing aids.
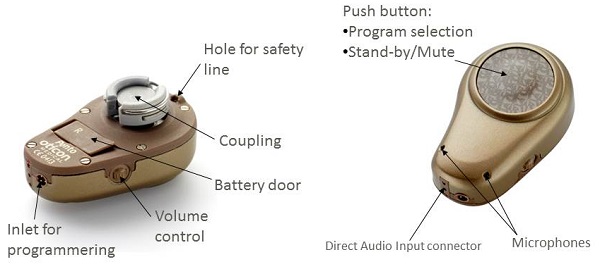
Figure 9. Ponto Pro sound processor and features.
Powerful
Percutaneous bone anchored processors gave the most power, resulting in the most gain. Direct bone conduction is the most efficient energy transfer to the skull. The Ponto Plus Power is the most powerful processor available, as shown by comparison in Figure 10. This is the frequency response from the Ponto Pro, Cochlear BAHA 4 Connect and Ponto Plus Power. The MFO is the maximum force output. Because it is a vibratory device, we are dealing with forced output.
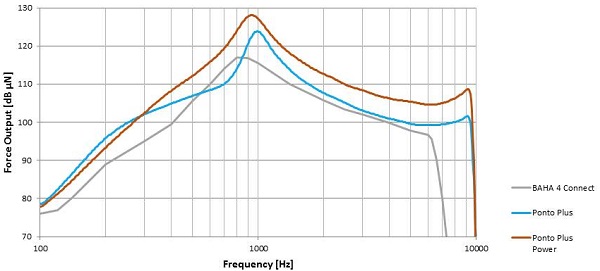
Figure 10. Maximum force output (MFO) comparison between BAHA 4 Connect (gray), Ponto Plus (blue), and Ponto Plus Power (orange).
Wireless Connectivity
Wireless connectivity to Bluetooth-enabled devices is now possible in the Ponto Pro Plus device through a streamer that you wear around your neck. The streamer connects directly to the cellphone, Tele Loop, MP3 player, and computer. The integrated telecoil enables you to connect to Tele Loop directly; the other devices require an accessory adapter to transform the input signal into a Bluetooth device signal, which the streamer can pick up and relay to the devices. You can also use a remote microphone or an FM system with the streamer, as well as an office phone.
Figure 11 shows the features of the streamer. By pressing the buttons, you can directly connect to the phone, microphone or the TV as part of the ConnectLine accessories. After you connect once, the streamer will automatically connect in the future when the hearing aids are in range. The battery charger connects with a mini USB plug, the FM system via EURO-pin socket and a range of audio devices with a mini jack.
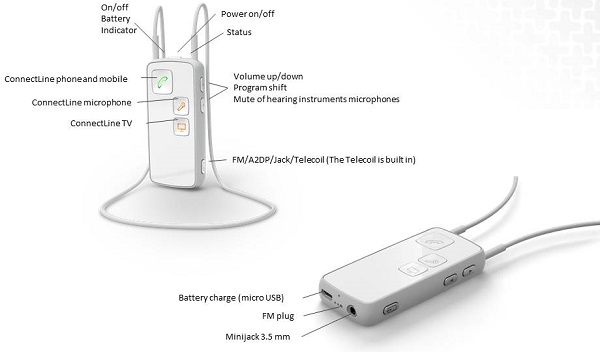
Figure 11. Streamer from Oticon Medical.
Skull Simulator
We have the ability now to measure the output of a bone anchored processor in a skull simulator. A skull simulator used to be bulky and expensive and were most often used by researchers in large organization.
The Interacoustics SKS skull simulator (Figure 12) works seamlessly with Interacoustics Affinity real-ear hearing aid analyzer system. It is used for the same purpose as a 2cc coupler is used for hearing aids. If the patient comes back with complaints, the first thing you want to do is test the device against specifications before you send it away for repairs. In the future, I believe we will have a real-ear measurement equivalent for bone anchored processors. This means we will be able to look at the output and also individualize each fitting to its unique skull and ensure targets are being met.

Figure 12. Interacoustics SKS skull simulator.
Transcutaneous Bone Anchored Solution
Again, in transcutaneous bone conduction technologies, there is the active, direct-drive with the vibrator implanted, and there is passive, skin-drive, where the magnet is implanted but the vibrator is still outside.
Figure 13 is an example of the devices that are currently available in the United States There is a Sophono system (left) and BAHA Attract (right). On the right side, you see an illustration of how the vibrator sits on the skull. The vibrations are transmitted across the skin to a magnet on either side that holds the components together. The sound is usually a little better than a processor attached to a Softband. It is virtually the same as passive transcutaneous solutions. You will lose up to 20 dB in the high frequencies, and there is a critical holding force of the sound processor as well. Some patients require a very strong magnet, which can result in some skin issues. The price is about the same as a percutaneous solution.

Figure 13. Passive transcutaneous skin-drive options: Sophono (left) and BAHA Attract (right). Sources: Sophono.com and Cochlear.com
Skin dampening measurements are shown in Figure 14. This shows the percutaneous solution versus the transcutaneous passive solution including the change in vibrator working points. You will lose quite a bit in the low and high frequencies because of skin dampening effect.
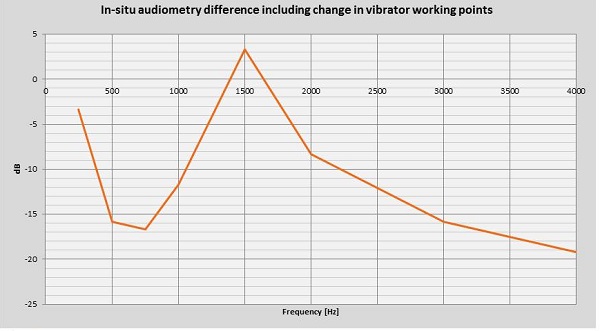
Figure 14. In-situ audiometry difference between percutaneous and the transcutaneous passive solution, including the change in vibrator working points.
Active Transcutaneous Solutions
The MED-EL BONEBRIDGE and the Bone Conduction Implant (BCI) are active transcutaneous solutions, and are not available in the United States. With these systems, the sound processor does all the processing of the signal and then it transmits the sound across to the inside through magnetic conduction. The signal is then transferred down to the transducer, which vibrates. Because it is implanted close to the cochlea, there will be some transmission loss in the high frequencies, however, not as significant as the passive transcutaneous solution. The position of the transducer to the cochlea does allow better high-frequency amplification.
The inductive link that transfers the signal across to the inside from the outside will lose some energy, but much of it will be recovered as it is close to the cochlea. A lot of energy is not required to vibrate the cochlea because the transducer is very close to the cochlea as opposed to being on the skin. There is increased sensitivity for the ipsilateral cochlea in the mid and high frequencies.
Today, we do not have the kind of battery power that would enable us to transmit low frequencies efficiently. You still get low-frequency amplification, but the power is not sufficient to transmit low frequencies in a robust manner. The whole skull has to vibrate to achieve this. In the higher frequencies, the vibrations are more localized.
The price of this system is about two to three times that of a percutaneous system, because most of the components are implanted inside. The size of the transducer could be a limiting factor. The MED-EL BONEBRIDGE cannot be placed in children due to its size; there has to be a large mastoid space or temporal bone to accommodate that transducer size.
The BCI, on the other hand, has a much smaller transducer. It is the brainchild of Eeg-Olofsson and Håkansson. Dr. Eeg-Olofsson is the creator of the surgery for the BCI. The inventor of the BCI device is Dr. Hakansson, who is also credited with the invention of the original bone anchored sound processor over 30 years ago. The BCI implant is smaller than the BONEBRIDGE in terms of length, depth, and size. There is a good review of these devices and others in Reinfeldt, Håkansson, Taghavi, and Eeg-Olofsson (2015).
Figure 15 shows the differing fitting ranges of the percutaneous and transcutaneous systems. The ipsilateral percutaneous solution will give you a fitting range up to 55 dB. If you are comparing that with the active transcutaneous system, low-frequency gain will be a challenge, but the high frequencies look good. The passive transcutaneous system loses more of the high frequencies and some in the low-to-mid region.
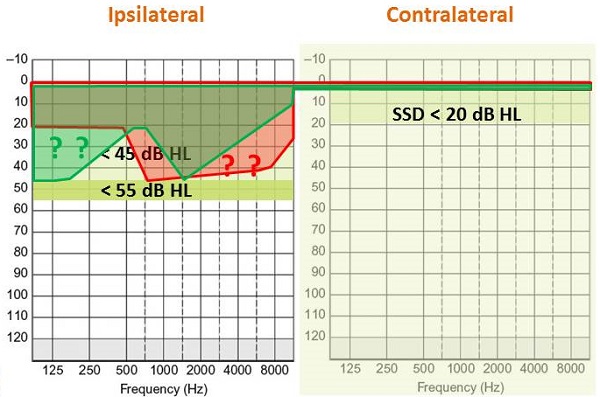
Figure 15. Fitting range comparisons of percutaneous (light green), active transcutaneous (red) and passive transcutaneous (dark green) systems.
Cochlear Implants
Cochlear implants are vastly different from middle ear implants and bone conduction implants. Cochlear implants work on electrical stimulation. An external sound processor takes the acoustic energy and transmits it to a coil that sits on the skull. The signal is then transferred across the skin to an implanted receiver, which then sends electrical stimulation across an electric array within the cochlea, tonotopically organized to stimulate different parts of the cochlea. The energy from the cochlea is then transferred up the cochlear nerve to the brain and perceived as sound.
The indications for cochlear implants vary by country, but generally are for adults and children suffering from bilateral, severe to profound sensorineural hearing loss (> 70 to 90 dBHL). These patients have tried conventional hearing aids with no functional benefit. Intelligibility is usually between 30% and 60% at 65 dB with hearing aids. There should be no medical contraindications, malformation of the ear, or psychological disorders. The patient should be highly motivated and should have realistic expectations. Also importantly, they should be able to attend numerous fitting sessions as there is intensive speech habilitation for children and rehab for adults. Much of this habilitation and rehab involves auditory-verbal therapy and speech therapy.
In children with prelingual deafness, cochlear implant candidacy is established when auditory skills fail to develop after traditional amplification and aural rehab over a three-month period. Implantation will usually be done after 9 to 12 months. Bilateral implantation is becoming more common in developed countries. The advantages are better localization, better speech understanding in noise, and also better balance perception in children.
Figure 16 shows an overview of what the cochlear implant process. After the hearing loss diagnosis is made, the hearing aid trial begins. Following a period of three to six months with no shown speech perception benefit, candidacy selection begins, followed by surgery. Activation on the implants happens about two to four weeks after surgery and is followed by habilitation.
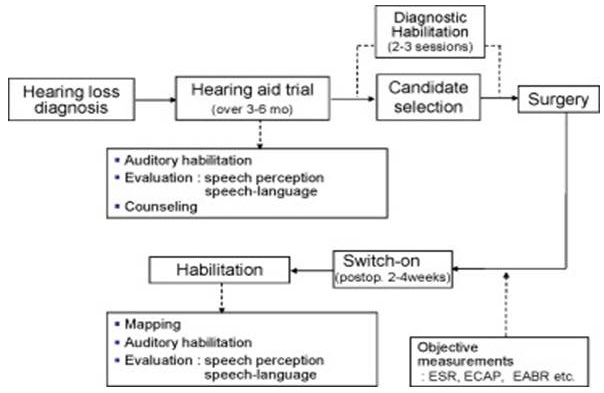
Figure 16. Cochlear implant process.
Outcomes
The complication rate is about 3% for cochlear implants in terms of failures, infection, and migration. There is wide variability in results as well. Generally, adults with the shortest duration of deafness experience better outcomes. The younger a child with congenital deafness is implanted, the greater the benefit. This has been well-established.
There have been advances in cochlear implant systems. Surgeries are becoming less invasive. With current implants, we do not have to do bed drilling. There is easy insertion of the electrode array. There is also a binaural system that is available, although it is not available in the United States; they are being used in Europe and Canada. You would have one receiver and one stimulator, two electrode arrays and two microphones - one left and one right. There is also only one speech processor. This is a very cost-effective alternative to bilateral implantation two implants and two processors.
Figure 17 explains how you have one speech processor and on the other side you have a microphone to pick up the sound from the other side. That sound is fed into the second microphone port of the speech processor. It does parallel processing for the inputs from the ipsilateral and contralateral side. There is one implant and one receiver, but two electrode arrays, one tunneled across to the contralateral side. The microphone picks up the sound, but puts it through to the second microphone port of the speech processor, and then it is fed into the electrode array that is on the contralateral side. This has been done in many patients in Central America, Europe, and Canada.
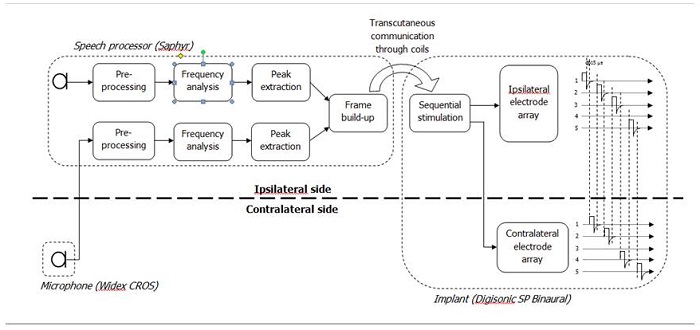
Figure 17. Binaural cochlear implantation.
Each microphone corresponds to one electrode array. The sound is routed to the corresponding electrode array to restore stereophonic perception. There is one speech processor and one implant with two electrode arrays.
Auditory Brainstem Implants
There are many indications for the auditory brainstem implant system. Some of these include neurofibromatosis 2, total ossification of the cochlea, and congenital aplasia. These are contraindications for a cochlear implant. The patient then potentially becomes a candidate for an auditory brainstem implant. There is an electrode array with 15 flat stimulation electrodes attached directly to the brainstem. Each electrode stimulates a different region so they can hear a wide range of sounds. It looks like a cochlear implant, but it is implanted in the brainstem.
The Future
What about the future? Cochlear implants are a well-established intervention for profound sensorineural hearing loss. They are also increasingly being used for single-sided deafness in Europe. Some patients in the United States have been implanted with cochlear implants for single-sided deafness as well.
Middle-ear implants, especially for those indications approved by FDA, are used for moderate to severe sensorineural hearing loss. Outside of the United States, some of these implants have been used for conductive hearing losses and mixed hearing losses. Percutaneous and transcutaneous solutions will still be seen. They will not disappear because they do provide good audiological outcomes, especially when surgery is simple, straight forward, and constantly evolving. We will still continue to see them being used for mixed hearing loss, conductive hearing loss, and single-sided deafness.
There is also a trade-off between cosmetics and audibility. The percutaneous solution for mixed conductive hearing loss will provide the best audibility and sound quality. If you want more in the way of cosmetics, then you have to compromise on the audibility by choosing one of the transcutaneous solutions.
Questions and Answers
What is the occlusion effect with the Maxum implant?
That is a good question. It seems like there is a hearing aid in the canal, although the manufacturer says that it is like open fit with not much of an occlusion. I would tend to think that there will be some occlusion effect, but it is something to ask the manufacturer.
Do you think one speech processor is a good idea for bilateral implantation?
That is a very good question. With the current technology, they are using two microphones. One microphone is being used for input from one side and the other microphone is being used for input from the other side, which I think is not the ideal thing to do. I would envisage technology would actually progress and advance so in the next couple of years or so, we will see much better sound processor technology. You may still have one speech processor, but the microphone on the other side is going to wirelessly connect to the speech processor. That might happen.
References
Reinfeldt, S., Håkansson, B., Taghavi, H., & Eeg-Olofsson, M. (2015). New developments in bone-conduction hearing implants: a review. Dovepress, 8, 79-93. DOI: https://dx.doi.org/10.2147/MDER.S39691
Cite this Content as:
Sockalingam, R. (2015, February). Implantable auditory technologies. AudiologyOnline, Article 13250. Retrieved from https://www.audiologyonline.com.


