My name is Ravi Sockalingam, and I am the Director of Clinical Research and Professional Relations with Oticon Medical based in the United States. Today, I will briefly go over what a bone anchored hearing system is, what it comprises and what it does, and what the candidacy prerequisites are for the system. Then I will go on to speak about the sound processor considerations. Development of the design and technology of the sound processor has advanced, particularly in the last few years. I will then talk about surgical considerations. There have also been several advancements in the surgical techniques and approaches that are being used now, even in children. Lastly, I will talk about what the future is going to look like for bone anchored technology, and what we can expect to see in the next three to five years.
As we know, bone anchored hearing systems have been around for almost three-and-a-half decades. They have been proven to be a very effective rehabilitation solution for conductive, mixed, and unilateral sensorineural hearing losses, often referred to as single-sided deafness in the literature, or SSD. They have also proven to be very safe in several long-term studies. The system’s clinical longevity is a testament to its long-term safety and success.
Candidacy
Mixed and Conductive Hearing Loss
Candidacy indications are for children five years or older. Bone-conduction pure-tone averages (PTA) have to be 45 dB or better, and the speech discrimination score should be greater than or equal to 60%. Additionally, there has to be general symmetry between the air conduction and bone conduction thresholds. By that, I mean there has to be less than a 10 dB difference between the PTAs for the two ears, or less than a 15 dB difference at any individual frequency of 500 Hz, 1000 Hz, 2000 Hz, and 4000 Hz.
Single-Sided Deafness
The age criterion is the same for SSD as it is for mixed and conductive hearing loss. According to the Food and Drug Administration (FDA), the definition of SSD is when you have profound sensorineural hearing loss in one ear and normal hearing (PTA threshold is 20 dB or better) in the contralateral ear. That definition encompasses the candidacy criterion for this device.
Off-label Considerations
Many people ask us why the age limit is five years and what other options there are for children under the age of five. In some countries, children are still surgically implanted if they are less than five years old; children as young as two-and-a-half or three years have been implanted in Canada. The FDA does not allow surgeons in the United States to surgically implant children under the age of five years. These children can still use the processor on a softband, which is placed around the head with the processor fit snugly against the skin on the mastoid bone. They will not be implanted with the titanium implant, nor will they have an abutment. Children can still benefit significantly from bone-conduction hearing through the Ponto processer worn on the softband until they reach the age where implantation is appropriate.
Figure 1 shows what a bone anchored hearing system looks like. Sound vibrations are transmitted by bone conduction via the skull to the bony portion of cochlea. On the right side, there are two pictures. What you see on the left is where the titanium implant has been imbedded within the skull and the abutment has been fixed. The sound processor, or the hearing aid part of the bone anchored hearing system, is then attached to that abutment on the outside of the head. The two pictures on the right-hand side you how the bone anchored hearing system looks without the sound processor (left-side picture) and also with the processor loaded on to the abutment (right-side picture).
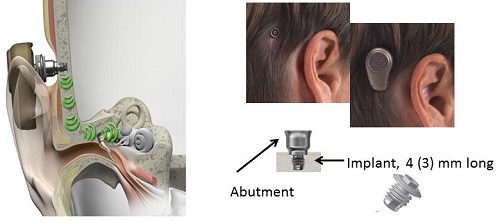
Figure 1. Bone anchored hearing system, where sound is transmitted by bone conduction via the skull to the bony cochlea.
The bone anchored implant is based on the concept of bone conduction and osseointegration, where the implant fuses with the bone tissue and becomes almost as one. In the case of SSD, there is profound sensorineural hearing loss on one side but a functioning, healthy cochlea on the other side. If you were to put a bone anchored hearing device on the side with profound hearing loss, the vibrations are transmitted through the entire skill to both cochleae, albeit with a very small delay to the contralateral side. You would still hear the sound through bone conduction.
We can actually use the bone anchored device as a CROS (contralateral routing of signal) hearing aid. Instead of using an air conduction CROS aid, you could use this device and not have anything occluding the good ear. By having the titanium implant imbedded in the skull with the abutment, you could transmit sound directly to the cochlea on the healthy side. This is how patients with SSD benefit from a bone anchored hearing device. There are many clinics in the United States where up to 70% of the patients fitted with the bone anchored hearing devices have SSD.
Figure 2 shows what the bone-anchored hearing system looks like when broken down into its various components. On the far left-hand side, you see the sound processor. There is a coupling system that enables the sound processor to be attached to the abutment. The abutment acts as a bridge to connect the sound processor via the coupling system to the titanium implant. The three main parts are the titanium implant that osseointegrates into the skull, the external abutment that connects the implant to the processor, and the sound processor that attaches to the implant and rests externally on the skull.
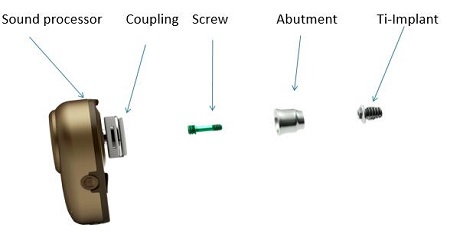
Figure 2. Ponto bone anchored hearing system.
Figure 3 illustrates the concept of direct bone conduction. The sound waves from the sound processor are transmitted directly via the bone to the cochlea.
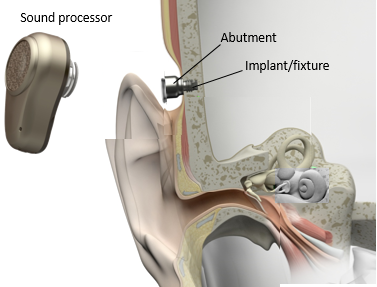
Figure 3. Direct bone conduction, from the sound processor, across the abutment, and directly into the bone of the skull.
Success of the Bone Anchored Hearing System
The success of the bone-anchored hearing system has been well-established. One retrospective review looked at 218 patients with bone anchored system (Wazen, Young, Farragia, Chandrasekhar, Ghossaini, et al., 2008). They found no major wound complications in any of the subjects. Because this device involves an abutment that has to pierce through the skin, you can expect minor skin complications in some of these patients. Any subject who had a minor skin complication was locally treated; there was no need for revision surgery. The implant extrusion rate was very low at 1.8%; this is the rate at which the implant comes out of the skull, also referred to as the failure rate. That number refers only to children.
Other studies have suggested a higher extrusion rate, up to 15% or 20% in some cases. That is mainly because of trauma that children incur due to their very nature of play and environmental exploration. They may fall when they engage in sporting activities, or something may hit their abutment, and there is a high likelihood that the implant may extrude or fall off. The study also revealed that the mean use of the device was 10 hours a day and 5.6 days a week. This is a testimony to the success of the bone anchored hearing system. It was also a study that was done in a larger cohort of patients; other studies tend to be very small.
Advancements for Pediatrics
We have seen many new developments in the field since the publication of that paper in 2008. There have been many advancements in the design and the signal processing of sound processors, particularly Oticon Medical’s Ponto. When Oticon Medical came onto the bone anchored scene, they were able to bring many advanced hearing instrument features with them. There have been advances in implant and abutment design and in surgical techniques.
There are many different considerations for children. Manufacturers have a responsibility to make a device that is compatible with their anatomy along with software and features that allow the audiologist to fit these patients with ease and accuracy. We have to take into account the children’s’ perspectives, because there are certain requirements that we have to fulfill for them to be able to use these devices consistently.
For a bone anchored hearing device to be child-friendly, I believe it has to be aesthetically appealing to the child who is using it and easy to use and handle for the parent as well. For very young children, the parents most often handle the device for the child. Older children can be trained to do those manipulations on their own. This device has to perform well in many different listening situations. It has to be robust and reliable as children are going about various physical activities. They should not be able to easily lose the device. If it falls, it should not crack and fall apart. They are going to be using it in a variety of weather conditions, from rain and snow to hot, humid conditions. These are all the considerations that are very important from a child’s perspective. The most important consideration is that it has to be safe. It will also have to be able to provide different communication possibilities. From an audiological perspective, it has to be easy to handle and easy to fit.
Design
A lot of thought has to go into the design of the system. The design is not just how the device looks, which is important, but it has to work the way it should for the child. It has to meet the needs of the child. The push-buttons, program selectors and mute button are very important things. The child must to be able to connect a FM receiver or a telecoil to the processor. Older children should be able to easily manipulate a volume control on their own. Tamper-proof and safety features for small children must be integrated. Such features include a safety line attachment and a tamper-proof battery door. The coupling system also has to be robust, because this is a device that is going to be put on and taken off several times a day.
The aesthetics has to be designed for children. It has to be shaped to blend in well. It is good if you have a right and left version, stickers to make the sound processor more personal, and different softband colors (Figure 4). The processors come in four colors: white silver, chrome beige, mocha brown, and diamond black.


Figure 4. Aesthetic features of the Ponto system. From top: right and left processors, personalized stickers on the processors which come in four different colors, and softbands available in six colors.
Ease of use is essential. The softband has to be elastic and adjustable to fit different head sizes. If you cannot adjust the band, you cannot fit it on different children. There needs to be a large and easy-to-find mute and program button for small fingers. Advanced signal processing with automatic features is a big benefit to users. The device should be programmable such that the child does not have to do any manual manipulation; everything should work automatically. Most hearing aids have done that for some time. Until three years ago, automatic features in bone-anchored devices were virtually unavailable. This is a big advancement.
Signal Processing
In terms of signal processing, we now have automatic multiband adaptive directionality. The hearing aid will go seamlessly from omnidirectional to full directionality to split directionality, depending on the signal-to-noise ratio of the environment in which the child is present. There is also wind noise management, which is a very useful feature for children and adults alike. When the child is wearing the instrument on the street, it becomes very difficult to carry on a conversation or hear important safety warnings in a windy condition. Wind noise management reduces the deleterious noise in these windy conditions.
The Ponto processor employs the SpeechGuard amplification scheme. For those of you who are unfamiliar with Oticon hearing instruments, SpeechGuard is the amplification scheme that preserves the natural sound quality of speech. We are now able to offer that in the Ponto, Ponto Pro, and Ponto Pro Power. Advances in feedback management systems allow us to customize the feedback solutions offered in our processors. We are able to measure the individual feedback limit for every child and set the appropriate gain below that limit. In summary, performance of the signal processing is important in order for the device to perform in a variety of listening situations, some of which are quite challenging.
Safety, Robustness, and Reliability
Safety, robustness and reliability are the most important factors, especially for children who are running around or participate in sports; trauma is a primary concern. If there is trauma to the sound processor, it dislodges itself from the abutment without damaging the abutment or the implant. It is able to absorb the impact and then come off.
The tamper-proof battery drawer is an important pediatric feature, especially to parents. The safety line attaches the device to clothing so that they do not lose it if they take it off. Nano coating protects the device from moisture and dirt entering into the casing.
Designed for the Audiologist
The Ponto bone anchored system was designed with the audiologist in mind. We have a dedicated softband fitting mode in the fitting software, although you do have to be sure to select the softband checkbox in the software. It will adjust the gain appropriately. In a softband fitting, the sound is transferred across the skin, as opposed to the transcutaneous implant. There will be some sound transmission loss across the skin, and we have to compensate for that with gain adjustments. That is all done when the audiogram is stored in the software.
Making in-situ bone conduction thresholds was an important development because we are not able to measure bone conduction thresholds directly off the abutment. When you use the traditional pure-tone bone-conduction vibrator, there is going to be some soft tissue vibration as well as bone conduction, and then some of the sound is going to be attenuated across the skin. With direct measurement, we are now able to prescribe a very accurate gain for the child when the child is wearing the device on the abutment as opposed to a softband. Being able to measure bone conduction thresholds directly off the abutment was a very positive step forward, because we have seen differences up to 20 dB in some cases. Sometimes we under-prescribe or over-prescribe when we rely solely on traditional bone conduction thresholds. Now with in-situ measurements, we will be able to prescribe more accurately.
Measuring the static feedback limits is an important step and is very unique to each patient’s head. Individualized static feedback control coupled with feedback cancellation makes for a very powerful feedback management system. Audiologists can make these measurements in a few minutes. We are able to adapt the device to the child’s needs. We can include special programs and we can also deactivate features, such as the volume control, if you do not want the child to have access to change certain features. Maybe you want to deactivate directionality because you want that child to receive sounds from all around him or her. There is flexibility in how we can program for a child.
The accessories that we can use with the device for pediatric connectivity include FM receivers, an external telecoil and the audio adapter for MP3 players, personal devices, or computers. These are very important accessories for a school-aged child who needs to be able to communicate in a classroom situation.
Pediatric Surgical Considerations
Surgical implantation, according to the FDA, is done in children age five or older. Typically, it is done in two stages for children under the age of 10, but this can vary from clinic to clinic and from one surgeon to another. Children 10 years and older usually do not have a staged surgery because the child’s bone is dense enough to ensure a strong implant and abutment, but if the child is under 10 years, the bone is often too thin and you would have to put the implant in first, wait for osseointegration to take place, and then put the abutment in. The time between the implant and placing the abutment on the implant could be between three and six months, depending on the surgeon and the clinic. This is why it is called a two-stage surgery.
The 10-year mark is not a rule that the FDA has stipulated, but, rather, is a guideline that many surgeons use based on research and anatomical viability. Children who receive one-stage surgery typically get a 4 mm implant, and the implant and the abutment are inserted in one surgery, just like we do in adults. There are advantages to using one-stage surgery. The obvious advantage is that you are not subjecting the child to two surgeries and two doses of anesthesia. There are fewer visits to the doctor. Most surgeons would load the processor onto the abutment about three months after the surgery. These children are treated more or less like adults.
Bear in mind the complications are greater in the pediatric population than the adult population. This has been shown in many studies. These complications can be due to issues relating to wound care, compliance in keeping the surgical site clean, and exposure to activities that will subject the device to extrusion or failure.
One large-scale, longitudinal study was performed in the United Kingdom, (McDermott, Williams, Kuo, Reid, & Proops, 2009), evaluating a pediatric bone anchored hearing aid program. The most cases of implantation took place from 5 to 10 years of age, followed closely by those who were older than 10 years. The highest fixture failure rate (40%) occurred in the group who were less than three years, followed by the group who were three to five years of age (38%). The rate dropped dramatically, however, in the group that was aged 5 to 10 years (8%) and those older than 10 years (1%). This dramatic drops occurs because osseointegration has taken place and the risk of it coming out is much lower. This number is very much akin to numbers you would see with the adult population. Even since 2009 when this study was published, there have been further developments in the design of the implant and the abutment.
Implant
Most implants are 3.5 mm, but a wider implant is also now available at 4.5 mm diameter. It is still based on the proven machine-smoothed surface, and this is called a Branemark implant. The Branemark design has been shown in many long-term clinical studies to be safe. It is well-proven and well-established. We have taken the same design and made the diameter larger, which provides greater bone-surface contact. Compared to our 3.7 mm diameter implant, the 4.5 mm implant gives 72% more bone surface contact. It is better for osseointegration, and it is more stable. It has been shown, through some of the studies being performed right now, that the stability measures increase from day one and keep increasing thereafter. Some clinics are able to load the processor in as few as three weeks if there are no skin complications.
Surgery
While two-stage surgery has been the standard for young children for many years, there is some research suggesting movement towards single-stage surgery. There are surgeons who are not comfortable doing two surgeries, especially in younger children, and prefer to wait until children are 10 to 12 years before doing a single-stage surgery. Peter Roland and his colleagues from the University of Texas Southwestern Medical School (2013) did a retrospective review of 65 children, which included 75 bone anchored devices; some of the children were implanted bilaterally. Of those cases, 60 were single-stage surgery and 15 were two-stage surgeries.
Their main research question when reviewing these cases was, “Will single-stage surgery result in a greater incidence of complications, especially failure of osseointegration?” The results of their study were presented at a scientific meeting in Copenhagen last March. The percentages for skin overgrowth requiring revision surgeries, both in single-stage and two-stage surgeries, were not statistically different. The percentage of cases that experienced a failure to osseointegrate was almost the same between the two groups. In the single-stage surgery group, there was only one case with infection requiring explant; no cases were reported in the two-stage group. Roland (2013) concluded that a single-stage bone-anchored hearing aid procedure is a safe and effective procedure for all ages. It minimizes the need for a planned second surgery. It allows the device to be placed sooner. However, consideration should be given to staging children with craniofacial abnormalities due to high extrusion rate related to thinner skull bones.
Other developments have focused on the surgical technique. Traditionally, bone-anchored surgeries were done with semi-circular flaps. We started off using a dermatome to thin the skin and removing the hair follicles. Then we progressed to a linear incision, which was started in Holland; they have done almost 1,000 cases using the linear technique. Many doctors in the United States have embraced this technique, and they are finding that this technique results in good surgical outcomes. The linear incision could also involve taking soft tissue out. There is now a move towards using a 4 mm linear incision, using a minimal soft tissue reduction. The philosophy is that if you do not traumatize the tissue, you potentially have fewer skin complications. They try and preserve as much tissue as possible.
Other doctors use a biopsy punch technique. They make a 5 mm or sometimes 4 mm punch. They may not have a lot of visibility with a punch that small, but they are able to go straight down to the bone and take out very little to no tissue at all. There is no skin thinning. There is no soft tissue reduction.
The linear incision technique without tissue reduction or skin thinning and the biopsy punch technique are minimally invasive. They are also referred to as tissue preservation surgical techniques. To date, there is only one long-term study conducted in the adult population on minimally invasive tissue preservation (Hultcrantz, 2013). Dr. Hulcrantz presented her results in a bone conduction/osseointegration conference in England this past June. She showed that minimally invasive tissue preservation techniques result in quicker healing, fewer tissue complications and fewer infections.
A retrospective study on 34 children was completed recently by Aviya Lamis and Malou Hultcrantz (2013). The participants were divided into two groups: skin thinning and no skin thinning. When skin thinning was part of the operative procedure, the researchers found a high incidence of peri-implant infection, which is infection around the implant site. Loss of abutment occurred in four cases, while three cases exhibited a need to remove the abutment. There was skin overgrowth in six cases. Five subjects had fixture loss due to lack of integration at 3 months, and 12 children had numbness around the surgical site after 12 months. Skin thinning is a more invasive procedure.
When skin thinning or tissue reduction was omitted from the surgical procedure, the complications were very few or nonexistent. Only one child had a peri-operative infection and only one had a loss of abutment. This shows that surgical technique can make a difference. When you use less invasive methods, you achieve better surgical outcomes.
The pictures in Figure 5 show the 3-month post-operative outcome after a no skin-thinning, non-tissue reduction technique. There is no divot that you may sometimes see when the patient is implanted. Additionally, the hair follicles are not removed, so hair will still grow naturally around the abutment. This technique has been performed with adults for some time, and now they are doing it in children as well because the outcomes are favorable.
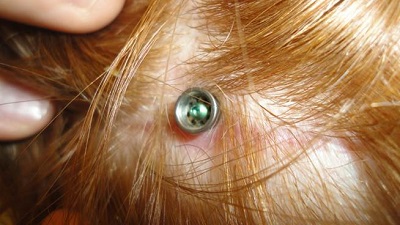
Figure 5. Three month post-operative surgical site after a minimally invasive implant procedure.
The developments in the surgical field are one-stage surgeries for younger children and using a minimally invasive approach. This means we have fewer skin complications, quicker healing, and, perhaps, sooner loading of the sound processor. In parallel, we have developments in the sound processor where we are seeing evolving technology, so we can provide better signal processing, better sound quality, better aesthetics, better ease of use and better software for audiologists to fit these devices.
Future
I do not have a crystal ball to know what will happen in the future, but I know what is currently happening, which can help us know what to expect in three to five years. I think we will continue to see developments in design in the immediate future, both in how the device looks and works. That also relates to signal processing. Signal processing will become more sophisticated, and the power consumption will be smarter, because battery power is as important for bone-anchored devices as it is for hearing aids. You will probably continue to see more developments in how feedback cancellation is handled. Feedback can still be an issue for people who need power instruments with more gain. In terms of transducer technology, we will see bone vibrators which are more efficient and can provide more power. They might even become smaller in size.
We will continue to see updates to wireless options. Patients will be able to connect these devices to Bluetooth-enabled mobile or land-line phones. They will be able to connect to the TV, FM receivers, and external or remote microphones. You will start to see wireless connectivity becoming a regular option for these patients.
The implant and abutment design will also continue to evolve in parallel with all of the developments in the sound processor. The geometry of the implant abutment has come a long way. With the smooth Branemark implant design, longer abutments and minimally invasive surgery, we are now attaining very good surgical outcomes. The geometry of the abutment where the skin meets the abutment surface forms a very good seal today. There is less risk of infection with bacteria pooling between the abutment surface and the skin interface. We will continue to see improvements in the geometry of the implant design and the abutment design.
Surgical techniques will also continue to advance. As more and more surgeons are embracing the minimally invasive technique, other advanced techniques will likely evolve. We will continue to see developments in the transcutaneous solution. Currently, we have percutaneous solution where the abutment sticks through the skin, but a transcutaneous solution is where we use magnets to transmit the sound across the skin. Part of the bone anchored hearing system would be inside the skull. The vibrator could be inside the skull very close to the cochlea, which is called an active transcutaneous solution. The vibrator could also be on the outside with the vibrations transmitted wirelessly or without an abutment across the skin to the skull, which is often called a passive solution. Either of these solutions could come into the marketplace in the next few years.
There is always a trade-off between the sound quality and the gain you can achieve with a transcutaneous solution. If you have a percutaneous solution delivering direct bone conduction, the gain that you are going to achieve is going to be higher. A transcutaneous solution, by design, results in energy or transmission loss across the skin. It could be up to 15 dB in the higher frequencies, depending on the solution you are using. The compromise is if aesthetics are an important factor in choosing the device, then a transcutaneous solution will be the option for this patient. If sound quality and gain is very important, then a percutaneous solution will still be the best solution for a patient. You will see developments in all of these areas in the near future.
At present, there is no way to verify these devices. In hearing aids, we can easily use a real-ear system to verify the output. We will continue to see developments for bone anchored systems in this area as studies are being conducted and tools are being developed so that audiologists will be able to verify these devices. Researchers are testing skull simulators to verify the output of bone anchored devices. A skull simulator is something that behaves like and has similar properties to the skull; it is similar to using a 2-cc coupler for hearing aids. Skull simulators used to be very expensive. Most clinics were not able to own one. Now we have a skull simulator that could be used with probe microphone systems. The skull simulator can only be used with the Interacoustics Affinity system at present. You can measure the output of the device as it leaves the factory or as it arrives in the clinic. Both the audiologist and manufacturer could check if the device is working according to specifications. The skull simulator is of immense value, because we can now objectively measure the output of the device. This is first step towards verification, in my opinion.
In the next two to three years, we will have specific prescriptive tools that will allow us to objectively measure how much sound the patient is getting in the skull. We could use those formulas to better prescribe and verify gain for these patients. We will see clinical applications of these tools in the near future.
Questions and Answers
Are there no long-term indications for the temporal bone for bone anchored hearing systems?
No. There are no long-term negative effects on the temporal bone. This surgery is actually reversible. You could take the implant out if the patient does not want to use the device after a while. Sometimes during the drilling in surgery, the surgeon can go into the dura, and in some cases there are cerebrospinal fluid (CSF) leaks. This is usually not a huge problem to resolve, even on its own. Doctors are well-trained to deal with that, and if it happens, it is not life threatening. It also does not affect the temporal bone.
Are there differences in gain that we should look for when fitting a patient who has SSD?
SSD assumes one normal ear, so what we are doing when we fit someone with single-sided deafness is routing sound to the good ear. The gain you are going to prescribe is to compensate for the transmission loss as sound crosses to the other side. There is usually a loss of energy, especially in the high frequencies; although it is very small, it happens. We tend to provide some gain to overcome that in the high frequencies. That is why we also tend to increase the gain in the high frequencies for that.
Are indicator lights a possibility with future devices? This is on many parents’ wish lists.
Yes. That is also on our wish list and something that we are definitely considering. It is a very useful feature to have, but one thing that we need to be aware of is the battery consumption. That is probably the stumbling block to implementation.
Is it still recommended to wait until after 12 months of age to fit a softband due to the skull differences?
No. This is based on my conversation with many practicing pediatric audiologists. They feel that the child is to ready to get a softband as young as five or six months. Most of this has to do with head control. When the child exhibits a bit of head control and when the softband with the device in place will stay on the head, it is often an indication that the child should be able to benefit from wearing it.
At what age would children with SSD be recommended for implantation?
There are two schools of thought on this issue. One group would say that we do not see any problems and the parents do not report any problems with children with SSD, so we do not usually intervene. On the other hand, we have literature that shows that these children are at a greater risk of having problems in school. The second group says we should intervene as early as possible with these children. Even if you intervene, how do you measure the outcomes and know that these children are benefitting from the device before they go to school? This is something that we need to investigate. It is a good question, and one of the many that are unanswered today.
There are a lot of questions relating to SSD in children. I am in the process of forming a pediatric alliance in bone anchored solutions. I am gathering pediatric audiologists from across the country, because they are a unique group of audiologists with specific expertise and perspectives from various clinics. The issues facing children are very different from those facing adults. There are many unanswered questions, and the hope is to have a group that can advise what the best practices might be. There are many areas where we do not any evidence, so part of the objective of the alliance is to ask these questions and work together to collect the evidence and come to a consensus on best practices in pediatrics.
References
Hultcrantz, M. (2013, June). A Non-skin thinning surgical technique: A five year follow up. Presentation at Fourth International Symposium on Bone Conduction Hearing and Craniofacial Ossointegration, Newcastle Upon Tyne, England.
Lanis, A., & Hultcrantz, M. (2013). Percutaneous osseointegrated implant surgery without skin thinning in children: a retrospective case review. Otology & Neurotology, 34(4), 715-722. doi: 10.1097/MAO.0b013e31827de4dd
McDermott, A., Williams, J., Kuo, M., Reid, A., & Proops, D. (2009). The Birmingham pediatric bone-anchored hearing aid program: a 15-year experience. Otology & Neurotology, 30, 178-183.
Roland, P. (2013, March). Single-staged bone anchored hearing aid in the pediatric population. Oticon Medical Scientific Meeting, Copenhagen, Denmark.
Wazen, J., Young, D., Farragia, M., Chandrasekhar, S., Ghossaini, S., Borik, M., Soneru, C., et al. (2008). Otology & Neurotology, 29, 1115-1119.
Cite this content as:
Sockalingam, R. (2013, October). Surgical and audiological considerations in pediatric bone anchored solutions. AudiologyOnline, Article 12225. Retrieved from: https://www.audiologyonline.com


